18th January 2019
Post by Tom Dredge, Senior PV Analyst.
pharmasol recently sponsored a leading industry conference where, amongst other excellent presentations, a discussion around Right-size PV really stood out.
The essence of the presentation was that when implementing a PV System in a new country or region, it must align with the capabilities of the overarching authorities otherwise there is a real risk that both the implementation and the ongoing activities will fail. So where, say, the EMA GVP might be seen as the Gold Standard, it may not be fully compatible with the available resources and competencies of that country or region.
Bottom line: You don’t always need a sledgehammer to crack a nut
But what if the Gold Standard is the only standard?
Now, in contrast to the establishment of a brand new set of regulations in a region or country – where beyond the guiding principles and generally accepted practices they are free to implement regulations as they wish – the implementation of computerized systems in the pharmaceutical industry, especially ones that support high-risk business processes such as a safety database, must align with robust and stringent guidelines and regulations already established by the various health authorities and regulatory agencies.
In addition, in order that the computerized system is fit-for-purpose in a global environment now and for the future, it must be compliant with the regulations in the largest markets. Therefore, it is important that the expectations of the FDA and EMA (and MHLW where appropriate) are met, especially as many other authorities and agencies use these regulations as a basis for their own.
Bottom line: The sledgehammer is always necessary when your nut is a PV Safety Database
So, how can we “Right-size” the safety database implementation?
Right-sizing is not necessarily concerned only with reducing the overall effort.
Right-sizing computerized system implementation is primarily driven by the make-up of the organization in question.
Let’s explore this further….
A typical project to implement or upgrade an on-premise safety database requires dedicated resources from across your organization, including PV; Business QA; IT QA; IT Engineering; IT Infrastructure; IT PM; and more.
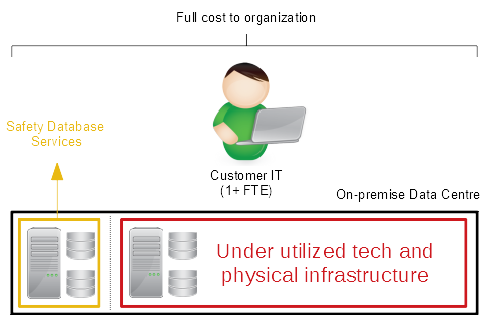
The ongoing maintenance and updates will then require some or all of that resource over the life cycle of the system. In addition, technology infrastructure (e.g. hardware) and physical infrastructure (e.g. a data centre) are required to deliver the system. And of course, all of the activities must be validated and controlled.
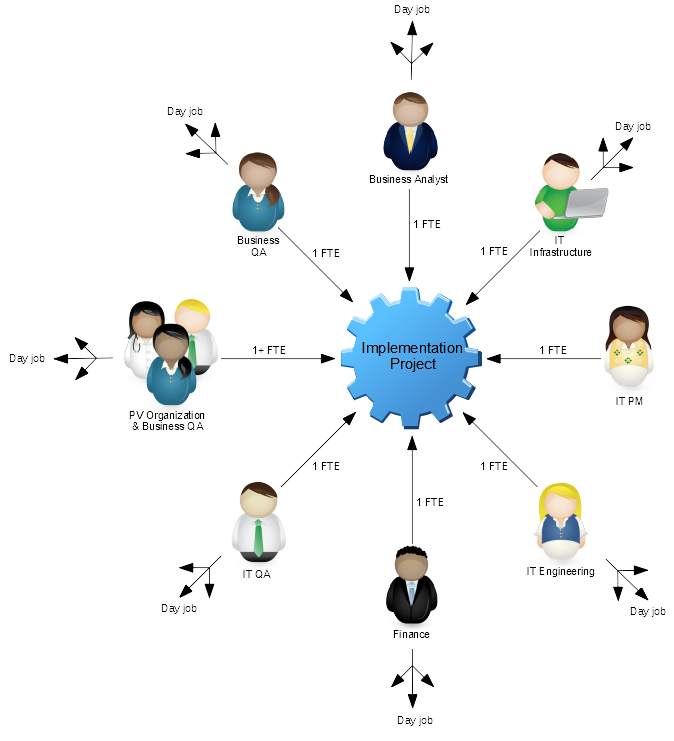
That is a lot of resource, time and financial commitment by anyone’s standards and can expose your organization to both financial risk (that may be unnerving, especially if the system is initially underutilised) and operational risk (by taking expert resources away from their core business roles for long periods of time).
Back to the question of “How can we Right-size the safety database implementation?”
The most practical, scalable and efficient way to Right-size the implementation of a safety database is to lessen the impact and risk to your organization.There are two main areas where that can be achieved:
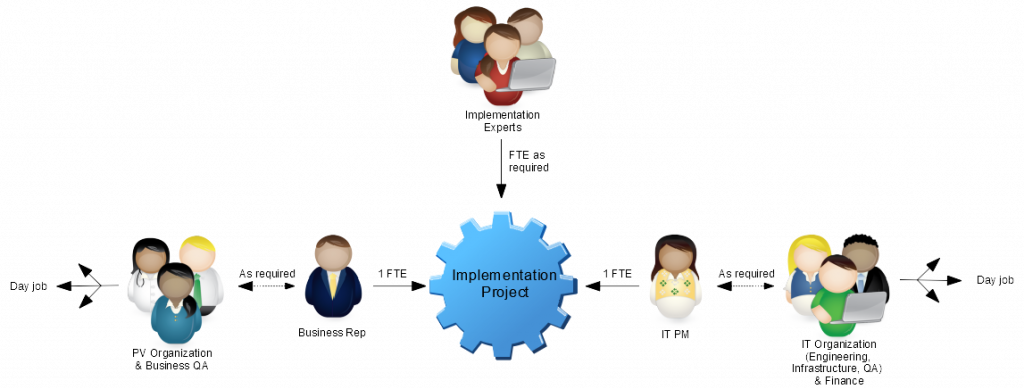
Bottom line: You can lessen the impact of the sledgehammer by utilizing external resources
That’s fine for the implementation project, but what about the maintenance phase?
Once a safety database is delivered (hopefully quicker, cheaper and better by utilizing industry experts), how can your organization continue to benefit from Right-sizing the maintenance effort?
The Maintenance Phase of a system is the major part of a system life-cycle. It’s the reason the system was implemented in the first place. It’s where it is used and upgraded and (sometimes) broken (and then fixed).
Traditionally, this part is executed by the business; Data Processing, Medical Review, Regulatory Reporting, etc. This generally forms the major part of the day-to-day activities for the PV organization.However, in order for the system to support the business, there is usually an administrative aspect; User Management, Study Set-up, Codelist Maintenance, etc. This business support function is generally very specialized – fitting into neither the IT nor PV areas. Consequently, safety database administrators tend to be hard to find and expensive to employ. In addition, in many small to medium sized companies, the administrator may either not have enough tasks to fill their day and/or have no back-up when they are absent or unavailable once again exposing the organization to undue financial and operational risk.
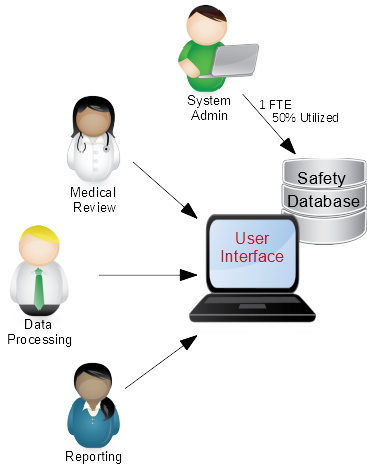
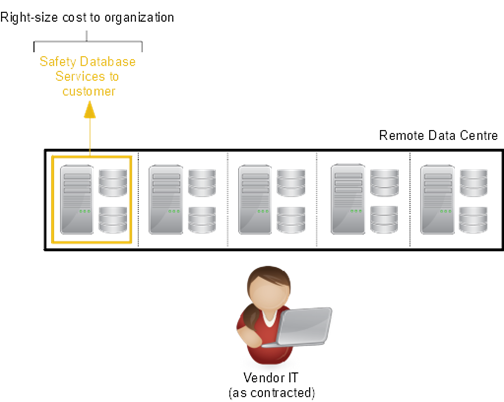
Right-sizing the administrative aspects of the safety database can be done in the same way as the implementation project; by lessening the impact to the organization.
We call this Right-sourcing – meaning to partner with an organization that already has fully trained and experienced staff for completing such tasks. This can be tailored across a number of models, including retainer and pay-as-you-go strategies, to establish a fully trained, fully available administrative partner that reduces the operational and financial risks.
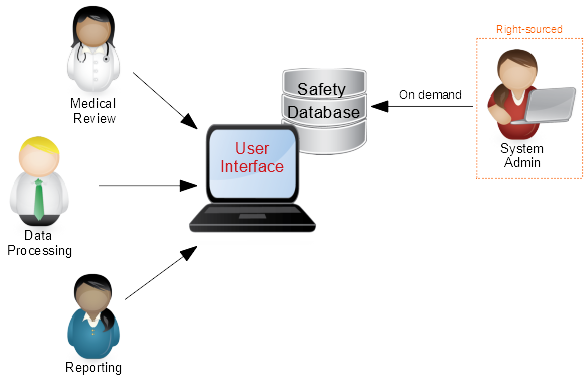
Bottom line: You can continue to lessen the impact of the sledgehammer by Right-sourcing
In Summary
A significant proportion of the overall risk associated with the implementation and maintenance of a safety database comes from the resource and infrastructure requirements.
Right-sizing the project by engaging with industry experts in technology, validation and processes can improve quality, speed of delivery and efficiency in the delivery of the project, and lower training, infrastructure and resource costs throughout the life cycle of the system.Finding a vendor who can dove-tail into your organization and provide a bespoke solution can be the key success factor in your safety database system implementation project.
pharmasol
pharmasol has developed rapidLIVE Argus and rapidLIVE Argus SaaS alongside their Expertise-as-a-Service/Right-Source and Consulting services to provide a Right-size offering for organizations looking to benefit from the market leading functionality of Oracle Argus Safety at a resource, quality, time and price point that supports a go-live that is as quick and as smooth as possible.
Tom Dredge is a computerized system validation and Argus professional who has worked in the Pharmaceutical Industry for 19 years.

22nd February 2024

25th September 2023

6th July 2023

31st January 2023

17th May 2022

8th April 2022

23rd February 2022

1st December 2021

1st June 2021

5th February 2020

8th March 2018