5th February 2020
Oracle introduced the Flexible Data Re-categorization (known as Flexible Code Lists) as part of the Argus 8 release.
“…there are potentially two places where updates to code lists must occur”
As the name suggests, the Flexible Code Lists allow for a greater degree of flexibility to store and maintain all types of code list values. Importantly, this code list data storage design is a single flat database table structure which is easier to maintain and can be leveraged to easily add new and custom code lists or values, without adding new database tables and columns.
As it stands, the traditional Argus Code Lists and Flexible Code Lists exist side-by-side and most of the Argus Code Lists are also available via the Flexible Code List – meaning there are potentially two places where updates to code lists must occur.

This may be important, as some code lists have parameters that can only be updated in one of the two places. An example is Causality Category which provides an EU Value in the Flexible Code List, but no equivalent in the Argus Code List. In order for the Causality Category value to be accepted by EudraVigilance, it must have an EU Value. So, in order to update the Causality Argus Code List, you must also ensure that the Flexible Code List is updated.
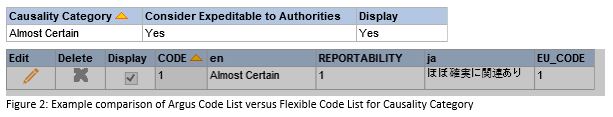
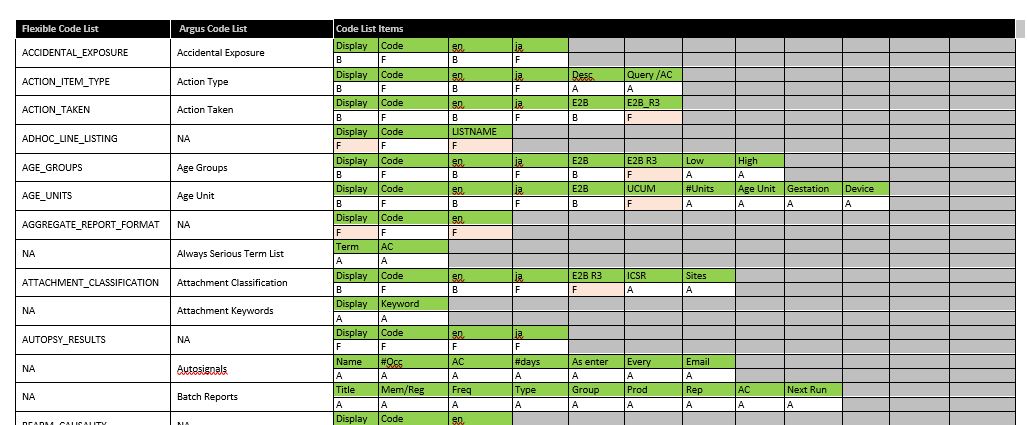
To mitigate some of the potential errors that can be introduced by having these two code lists existing together, pharmasol has created a detailed table (sample below).
In it, we list all of the default Argus Code Lists and Flexible Code Lists, the associated available fields for both and indicate whether that field is editable in the Argus Code Lists only (A), the Flexible Code List only (F), or both (B).
“…Flexible Code Lists…are the ones most likely to be overlooked”
As many of our customers work from the Argus Code Lists, we have specifically highlighted where a field is editable only in the Flexible Code List (orange/pink shaded cell with an “F”), as these are the ones most likely to be overlooked. Such a table is useful in supporting your daily Argus administrative activities.
If you would like a full version of this in Excel (which also includes additional columns and explanation of the different fields), or you have any questions about this or any other aspect of the Argus Safety suite of products, please get in contact using the form at the bottom of the page.

22nd February 2024

25th September 2023

6th July 2023

31st January 2023

17th May 2022

8th April 2022

23rd February 2022

1st December 2021

1st June 2021

5th February 2020

8th March 2018